Desenvolvimento de anticorpos IgY contra Streptococcus equinus cepa JB1
2 - School of Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo
RESUMO -
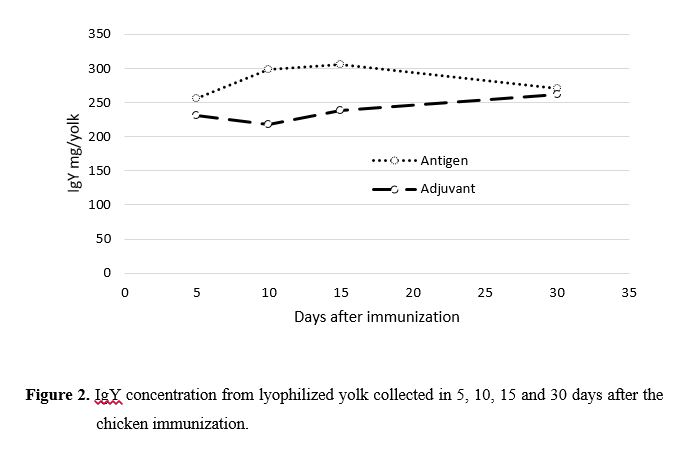
A Streptococcus equinus produz ácido lático e provoca acidose ruminal e pode ser controlado por anticorpos de galinhas. Poedeiras brancas Leghorn com 25 semanas de idade foram imunizadas com vacinas contendo Streptococcus equinus strain JB1 isolada do fluido ruminal. A bactéria foi ativada e placas de cultura por 24–48 horas a 37°C sobre condições semi-anaeróbicas. As colonias foram coletadas através de alçadas e transferidas assepticamente para tubos de 3 mL com solução salina. A solução salina contendo antigenos foi misturada (50:50) com adjuvantes (35 mL PBS mais 175 mg Al(OH)3). A mistura foi aquecida a 60°C por 40 minutos para inativação. A vacina foi transferida para tubos estéreis, fechada e estocada a 4°C até o momento do uso. 500 microlitros da vacina foi inoculada de forma intramuscular no peito de 80 poedeiras. Os ovos foram coletados diariamente de 5 a 30 dias após a imunização, quebrados e separados em casca, clara e gema. Depois a membrana vitelinica foi rompida e a gema foi submetida a delipidação e liofilizada. As amostras foram analisadas quanto a concentração de imunoglubulinas IgY usando um kit ELISA específico. Os níveis de IgY aos 5, 10, 15 e 30 dias após a imunização foram 256, 298, 306 e 271 mg/gema, respectivamente. Nós concluímos que a imunização com S equinus foi efetivo na produção de anticorpos.
Development of IgY antibodies on Streptococcus equinus strain JB1
ABSTRACT - The Streptococcus equinus is a major bacteria latic acid producer, cause acidosis and can be controlled by hens antibodies. Eighty 25-weeks-old white Leghorn hens were immunized with a vaccine containing Streptococcus equinus strain JB1 isolated from bovine rumen fluid. The stock culture was thawed and grown in blood plates for 24–48 hours at 37°C under semi-anaerobic conditions. Colonies were collected by scratching and aseptically transferring to a broth tube with 3 mL saline solution. The saline solution containing antigens was mixed (50:50) with adjuvants (35 mL PBS plus 175 mg Al(OH)3). The mixture was heated at 60°C for 40 minutes (as required) for inactivation. The vaccine was transferred to sterile serum bottles, capped, and stored at 4°C until further use. Five hundred microliters of the mixture were deeply inoculated in the pectoral muscles of 80 hens. Eggs were collected daily 5–30 days after the immunization, broken, and the shell, yolk, and egg white were separated. Then, the vitelline membrane was disrupted and the yolk was subjected to lyophilization and delipidation. After delipidation, samples of the yolk were analyzed for determining the concentration of IgY using the chicken IgY enzyme-linked immunosorbent assay. IgY levels at 5, 10, 15, and 30 days after immunizations were 256, 298, 306, and 271 mg/yolk, respectively. We conclude that immunization with the S. equinus JB1 strain was effective for antibody production.Introdução
Livestock production is the most significant activity of Brazilian agribusiness. The country contains the second largest herd of cattle (205 million heads) and is the second largest producer of beef worldwide (IBGE, 2010). The intensive raising of animals has increased the use of veterinary drugs and growth-promoting substances to maximize food production efficiency via the control of the ruminal microbiota. Monensin and virginiamycin are used commonly to eliminate Gram-positive and protozoan microorganisms associated with undesirable processes in the rumen of animals reared on pasture or in confinement. However, the use of antibiotics in animal feed might result in the selection of resistant microorganisms among the commensal bacteria and transient pathogens of the animal's gastrointestinal tract (Salyers & Whitt, 2005). This occurrence is associated with incorrect therapeutic doses or incomplete treatment and may represent potential public health problems (Russell & Houlihan, 2003). Certain bacterial strains isolated from cattle are resistant to multiple antibiotics and could be sources of potential human health hazard (Evans et al., 2005). Therefore, the European Union has banned the use of antibiotics as food additives since 2006 (Palermo, 2006). However, since the use of antibiotics is important for producing food of animal origin, efforts have been directed towards finding alternatives that can provide the same benefits as antibiotics along with food safety. Use of antibiotics alleviate metabolic problems due to lactic acid production, enhance animal performance, and increase production costs, albeit by compromising the productivity and competitiveness of livestock production in the country. The results pertaining to the use of polyclonal antibodies are contradictory. The administration of the liquid form of the antibody to large numbers of animals is impractical. In addition, Bastos et al. (2012) did not observe any effect on the molar concentrations of acetic acid, propionic acid, and butyric acid, total fatty acid concentration, acetic acid: propionic acid ratio, ruminal pH, levels of ammoniacal nitrogen and lactate, and protozoan population using diets containing 73% concentrate (based on dry matter) and spray-dried powders of the Camas Inc. polyclonal antibodies with increasing doses of 0, 1.5, 3.0, and 4.5 g/day. These conflicting results are probably due to differences in the strains and the drying process, and high variation in animal, diet, location, production system (in confinement or pasture), and physiological phase of the animal. In view of the increasing consumer demand for healthy and natural food, new feed additives that may improve the rumen environment by positive manipulation of fermentation are required. In this study, we develop the effects of a lyophilized avian-derived polyclonal antibody preparation on a specific ruminal bacteria, Streptococcus equinus JB1 (IgY-JB1).Revisão Bibliográfica
The bacterium Streptococcus equinus is responsible for metabolic problems (Schwartzkopf-Genswein et al., 2003) caused by fermentation of non-structural carbohydrates, mainly starch and sugars, and production of lactic acid. This bacterium dominates the intestinal flora when animals consume soluble carbohydrate-rich diet, resulting in low ruminal pH, metabolic disorders that cause acidosis, and poor animal performance. Several studies have demonstrated the potential of polyclonal antibodies in inhibiting the growth of unwanted ruminal bacteria. The efficacies of polyclonal antibodies in liquid form containing IgY against Streptococcus bovis (ATCC 9809), Fusobacterium necrophorum (ATCC 27852) and Escherichia coli (O157: H7) or Clostridium aminophilum (ATCC 43895) and Peptostreptococcus anaerobius (ATCC 49031) (Camas Inc., Minnesota USA) were compared to that of monensin by several researchers. Blanch et al. (2009) and Marino et al. (2011) observed higher ruminal pH and lower incidences of acidosis. Otero (2008) used antibodies in liquid form in the diet of crossbred Holstein × Bos indicus cattle, and observed an increase in the potential degradability of neutral detergent fiber NDF and an increase in the number of Isotrichan protozoa. Millen et al. (2010) and Pacheco et al. (2012) observed an increase in dry matter intake; however, they did not observe any differences in bovine blood parameters. Pacheco et al. (2012) reported reduction in rumenites, while Rodrigues et al. (2013) and Millen et al. (2009) observed no effect. Dilorenzo et al. (2008) used antibodies in the liquid form (2.5 mL/day) and observed higher daily weight gain, better feed efficiency, warm carcass weight gain, increase in subcutaneous fat thickness, and better carcass classification, whereas Pacheco et al. (2012) and Millen et al. (2015) found no difference using antibodies in liquid form or as spray-dried powders, respectively.Materiais e Métodos
Research on animals was conducted according to the guidelines of the institutional committee on animal use (protocol number 223-15). Eighty 25-weeks-old white Leghorn hens were immunized with a vaccine containing Streptococcus equinus strain JB1 isolated from bovine rumen fluid (a kind gift from Dr. Hilário Mantovani, Universidade Federal de Viçosa, MG, Brazil). The stock culture was thawed and grown in blood plates for 24–48 hours at 37°C under semi-anaerobic conditions. Colonies were collected by scratching and aseptically transferring to a broth tube with 3 mL saline solution. Turbidity was checked and compared to the McFarland standards to obtain a final density of 5 × 109 cells/mL. The saline solution containing antigens was mixed (50:50) with adjuvants (35 mL PBS plus 175 mg Al(OH)3). For the adsorption of antigens, the adjuvant was added slowly and gradually, followed by 4 hours of constant agitation. The mixture was heated at 60°C for 40 minutes (as required) for inactivation. The vaccine was transferred to sterile serum bottles, capped, and stored at 4°C until further use (up to 2 weeks). Five hundred microliters of the mixture were deeply inoculated in the pectoral muscles of 80 hens. Eggs were collected daily 5–30 days after the immunization, broken, and the shell, yolk, and egg white were separated. Then, the vitelline membrane was disrupted and the yolk was subjected to lyophilization and delipidation according to Akita & Nakai (1993). The lyophilized yolks were used as feed additive (8.5 g/d). After delipidation, samples of the yolk were analyzed for determining the concentration of IgY using the chicken IgY enzyme-linked immunosorbent assay (ELISA) kit (IRKTAH1109; Innovative Research Inc., Novi, MI, USA) and to monitor activity over time after the initial immunization.Resultados e Discussão
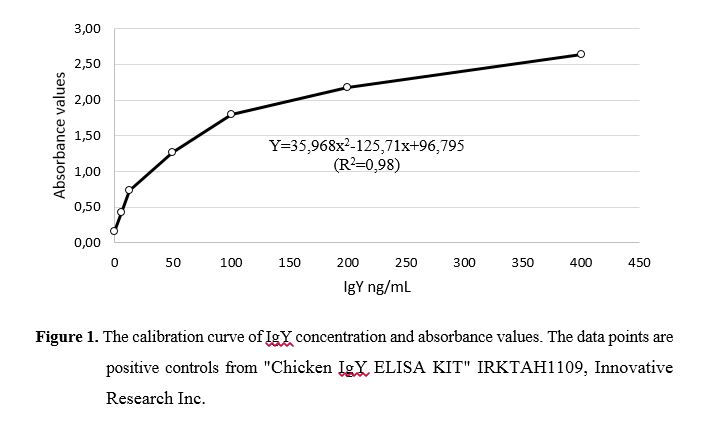
The calibration curve and IgY concentration for lyophilized yolk collected 5, 10, 15, and 30 days after chicken immunization are presented in Figures 1 and 2, respectively. IgY levels at 5, 10, 15, and 30 days after immunizations were 256, 298, 306, and 271 mg/yolk, respectively. Dilorenzo et al. (2008) used antibodies in the liquid form (2.5 mL/day) and observed higher daily weight gain, better feed efficiency, warm carcass weight gain, increase in subcutaneous fat thickness, and better carcass classification. However, the administration of the liquid form of the antibody to large numbers of animals is impractical. On the other hand Millen et al. (2015) found no difference using antibodies as spray-dried powders and Bastos et al. (2012) did not observe any effect on the molar concentrations of acetic acid, propionic acid, and butyric acid, total fatty acid concentration, acetic acid: propionic acid ratio, ruminal pH, levels of ammoniacal nitrogen and lactate, and protozoan population using diets containing 73% concentrate (based on dry matter) and spray-dried powders of the Camas Inc. polyclonal antibodies with increasing doses of 0, 1.5, 3.0, and 4.5 g/day. Considering that these differences may occur depending on the form of presentation (liquid or powder) and method of drying the products (spray-dried), we conclude that immunization with the Streptococcus equinus JB1 strain is effective for antibody production and the lyophilization could be a good way for preserving of heat-sensitive antibodies functionality.Conclusões
We conclude that lyophilization is an excellent method for preserving of heat-sensitive antibodies and that immunization with the S. equinus JB1 strain was effective for antibody production.Gráficos e Tabelas